
So if we look at 82, that corresponds the Lead or Pb, and so that will be the other particle that's produced and so you have in that decay. So each atomic number corresponds to a particular elements. Well which one will get that element number? Well you take a look at the atomic number, because remember each atomic number tells you elements rule.
#Nuclear fission equation plus#
And so, if we have 84 equals 2 plus blank, well 2 plus 82 gets me 84.Īnd the last thing is we want out what the element symbol is. They should also total each other on opposite sides. And then also the atomic numbers should also do the same thing. So that’s easy math, that should be pretty easy for everyone. So we say 206 equals 4 plus, blank, well easy math or algebra says that, 4 plus 202 will get us 206, because the yields sign is like the equal sign. So the mass number total each other on the opposite side.

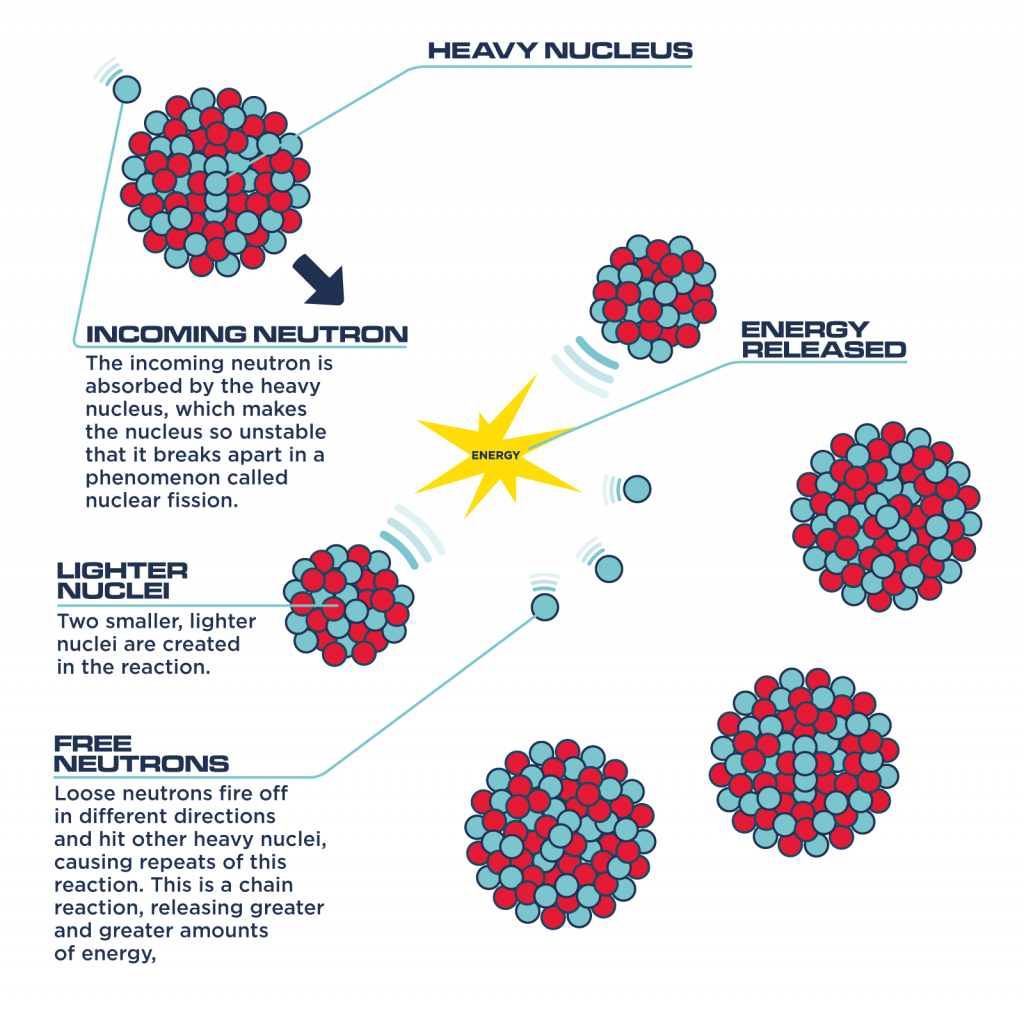
And then what we want to do is, we want to make sure that the mass numbers total each other on the opposite sides. So that mean that everything on the left side of the yield sign would be on one side, and then the yield sign is like an equal sign. So what we do is, the yield sign in chemistry, is like an equal sign in math. And then we want to find out what the other particle is. And iif we’re told that this undergoes alpha decay, then what we know is that we'll write 4 over 2 He. So in our first example that we have here, we have 206 over 84 Po. And so, basically what happens is, you have different types of decay and in nuclear radiation problems, you want to figure out what type of particles released or what the other thing is. So the charge will be +1, so it’s a positive electron. And so it’s actually zero over 1, so it has a mass number of 1 and has an e here. Positive, electron is actually kind of a misnomer. And so for those who are in English class you know what oxymoron is. And a positron basically combines two words positive and electron. And gamma radiation is the worst form of electromagnetic radiation that we know right now. And so a lot of nuclear explosions, or nuclear bombs, or others things like that release gamma radiation. And so as you know, gamma radiation is usually really bad. So you usually have zero over zero, because it has relatively very little mass and that’s the gamma sign. And so gamma radiation usually accompanies other decay processes. Or you might see it as 0 over -1 with a beta right here.Īnd then, the next set that you have there, is gamma. It has an atomic number of -1 because remember, electrons have a negative charge. And so, basically what you have is, you have a mass number 0, -1 and you have an e, because the beta particle is also known as an electron. You might see it as beta or beta negative. You also have another type particle called a beta particle. So enough particle you would know that that's a helium atom. So you have basically a helium atom with the mass number of 4, and then the atomic number is 2. And the first type is called an alpha particle, and an alpha looks kind of like this, with the letter right there. And so there are 4 main types of radiations that you’ll probably encounter in your chemistry class. While uranium-235 is the isotope that undergoes fission it is worth noting that uranium-238 atoms can absorb neutrons to become plutonium-239 which is another atom that can undergo fission.Here are some tips and tricks for writing nuclear radiations equations. We will consider where the energy comes from in the next section. It is 50 million times more energy than burning the equivalent amount of carbon. The energy released per fission is relatively large.The neutrons may need to be slowed down and are then referred to as thermal neutrons. Both the number and speed of the neutrons is crucial within a working reactor. For fission to occur the neutrons must be going at the right speed – too fast and they will bounce off rather than be absorbed.Once started, fission can become self-sustaining – this is called a chain reaction. The fact that neutrons are also produced means that these neutrons can go on to induce further fissions.These by-products of nuclear power form the majority of the radioactive waste that we will consider next week. Fission products tend to be radioactive.In words this would be: ‘A uranium-235 atom absorbs a neutron to become uranium-236 which then undergoes fission to form the products xenon-140 and strontium-93 with three neutrons.’ An equation representing this particular fission would be: The fission products themselves can vary but examples would be xenon-140 and strontium-93. Neutrons are shown as using the same notation as for isotopes. This image shoes the particles and types of radiation involved in fission Figure 14 The particles and types of radiation involved in fission


 0 kommentar(er)
0 kommentar(er)
